IonMed’s novel alternative to stitches, staples and sealants is well on its way to commercialization for applications including skin conditions.
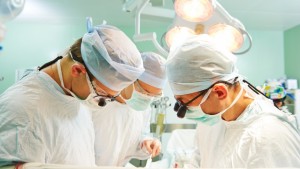 One year after receiving CE clearance in Europe for its Bioweld1 cold-plasma system for closing surgical incisions, Israel’s IonMed has completed clinical trials in Queen Astrid Military Hospital in Brussels — the largest burn center in Europe – showing the system’s advantages in skin-graft fixation over sutures, fibrin glue or staples.
One year after receiving CE clearance in Europe for its Bioweld1 cold-plasma system for closing surgical incisions, Israel’s IonMed has completed clinical trials in Queen Astrid Military Hospital in Brussels — the largest burn center in Europe – showing the system’s advantages in skin-graft fixation over sutures, fibrin glue or staples.
“Staples account for about 90-plus percent of the market. Fibrin glue is very expensive so although it’s good, many hospitals and clinics cannot afford to use it,” explains IonMed clinical manager Dr. Yaara Yarmut. “Staples create local pressure points that can lead to necrosis or infection. The advantage of our device is that it is cheaper than fibrin glue and more efficient than staples.”
No infections developed in the 20 burn patients involved in the IonMed study, and doctors reported achieving better closure rates than they do with staples.
“The investigator who worked with us was very pleased with the device,” Yarmut tells ISRAEL21c. “We hope to get CE clearance for this application during the first quarter of 2016. Right now the product can only be used to close surgical wounds, but after we get clearance it would also be used to close skin grafts and traumatic wounds.”
The startup, based in the Lower Galilee city of Yokneam Ilit, will next focus on the treatment of skin diseases including acne. Yarmut said cold plasma has characteristics that can change the mechanisms involved in the formation of acne lesions. “We’re working on performing a clinical trial in Europe for this application,” she says.
IonMed CEO Shai Levanon says cold plasma “holds enormous promise as a new therapeutic paradigm in wound care, and more broadly in medicine.”
In addition to sealing wounds, cold plasma speeds healing and has antimicrobial properties.
Until now, the company has been supported by its major shareholders, The Trendlines Group and Generali Financial Holdings Fund.
“That support allows our company to enter into expanding activity across the cold-plasma platform,” says Levanon. “However, to make progress in commercialization, especially after our success in the skin-graft trial, we are looking for additional investors to join us.”


